Public projects
MPM-HISTO (Histolution)
Multiphoton microscopy for section-free H&E histology
he aim of the MPM-HISTO project is to accelerate cancer operations and avoid further operations in order to increase patient welfare and save costs in the healthcare system. For this purpose, we are developing a microscope with which tumor tissue can be examined directly in the operating room without much effort, instead of first sending it to a pathology laboratory.
Challenge and Solution
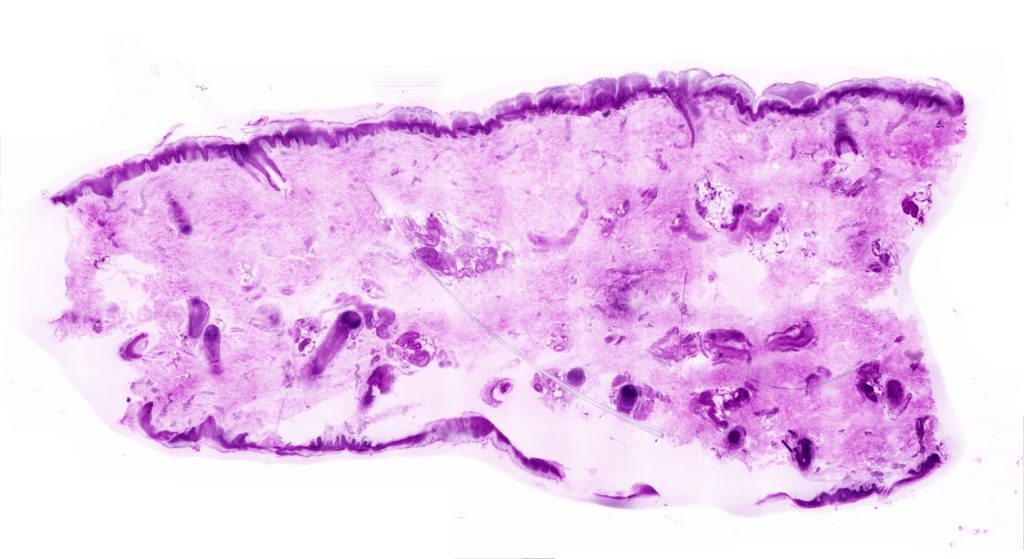
During tumor removal, the surgeon often cannot directly see whether the cancer has been completely excised. To answer this question, the excised tissue is examined microscopically in a pathology lab and feedback is given to the surgical team by telephone. In the pathology lab, the tissue is snap-frozen, cut into paper-thin slices, mounted on slides and stained with the dyes hematoxylin and eosin (H&E) before being viewed under a microscope by a pathologist. The examination can interrupt surgery for up to an hour.
For certain tumors, such as skin cancer, patients are even kept in the hospital overnight because a more complex examination method is required. In the so-called paraffin section, tissue is first drained and clarified overnight, then poured into wax, cut, mounted, stained and examined. This procedure usually takes 24 hours.
With our new microscopy method, the piece of tissue is only briefly stained in the operating room, scanned directly and then examined by a pathologist via telemedicine. As a result, the entire procedure can be shortened to less than 15 minutes.
Method
Instead of cutting the tissue, we use multiphoton microscopy to create a virtual section through the tissue. This uses the effect of fluorescence, which is also known from the black light lamp at the disco: a dye is illuminated and radiates back in a different color. The emitted photons have less energy than the irradiated photons. In multiphoton fluorescence, the dye is illuminated simultaneously with several photons of lower energy each, which add up to the energy actually required to cause fluorescence. At the focal point of the optics – and only here – enough photons meet at the same time to cause any bound dye to glow. Now the focal point is used to scan the tissue to generate an image pixel by pixel on the computer. The exact scan plane can be freely selected and 3D scans of the tissue can be generated without cutting the tissue.
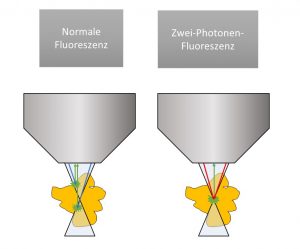
The technology of multiphoton microscopy has existed since the 1990s. As a rule, however, these are still room-filling setups that require costly maintenance. In contrast, our method is based on a specially developed, particularly robust fiber laser, which can also be used maintenance-free and compact in an operating room.
Planned Development
The laser and its application have already been tested in our laboratory. However, the setup is not yet operable by medical staff and our acquisition software is not yet optimized for high acquisition speeds. During the project period, a technology demonstrator optimized for clinical application will be developed and first systematic studies will be performed.
contakt: jan.kolb(at)uni-luebeck.de


