Public projects
Neuro-OCT
Optical coherence tomography in neurosurgery
2.3 million euros for new joint project – Extremely fast acquisition and visualization of tumor borders and vascular architecture during surgery
The Federal Ministry of Education and Research is funding a Lübeck joint project for three-dimensional, high-resolution real-time microscopy in neurosurgical operations. The goal is to remove tumors of the central nervous system more radically in future and to protect healthy brain tissue more effectively at the same time. It aims to prevent early recurrence and preserve a higher quality of life for patients.
Involved are the Department of Neurosurgery of the University Hospital Schleswig-Holstein, Campus Lübeck, the Institute of Biomedical Optics of the University of Lübeck and the Medical Laser Center Lübeck. Funding by the German Federal Government in the field of action Health Economy of the Framework Program Health Research Germany amounts to 2.291 million euros from October 2017 to September 2020. Head of the joint project is Dr. med. Matteo Mario Bonsanto, Department of Neurosurgery.
In Germany, about 43,000 new oncological diseases are diagnosed each year in the central nervous system (CNS). Frequently, a microsurgical tumor resection is indicated on the CNS. The more radical the neurosurgeon resects the tumor, the less likely it is for localized tumor recurrence to occur. The problem here is the often inadequate recognizability of tumor, vascular processes and tumor borders in contrast to healthy brain tissue. For tumor and tumor boundary imaging are usually surgical microscope and fluorescent dyes that accumulate in the tumor and make it visible under the surgical microscope with special filters and used the neuronavigation. As a further aid to tumor imaging, intraoperative ultrasound and intraoperative magnetic resonance tomography are used, if present.
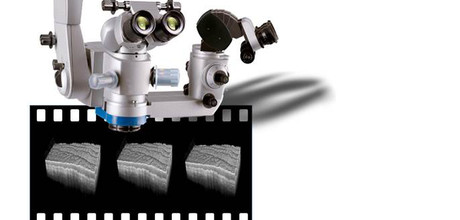
The Lübeck project aims to compare the current procedure of intraoperative fluorescence with two new different intraoperative imaging techniques, namely optical coherence tomography (OCT) (spectral domain OCT at 800 nanometers wavelength), which is already available as a prototype in neurosurgery, where the surgeon is shown a depth cross-section, as well as an innovative high-frequency OCT (MHz swept source OCT at 1300 nanometers wavelength), developed by Prof. Robert Huber, head of the subproject of the Institute of Biomedical Optics.
The extremely fast third-generation high-frequency OCT has the advantage that the entire resection space in 3-D can be displayed in real time. Due to the higher wavelength, one also has a significantly lower tissue spread, whereby a higher tissue penetration depth is achieved. In addition, the visualization of blood vessels (angiography) should be realized. In the clinical setting, both methods are evaluated for their sensitivity and specificity for tumor detection against fluorescence and validated for tumor cell detection with the gold standard, histology.
This task, together with the Department of Neurosurgery, will be carried out by Dr. Ralf Brinkmann and his team at the Medical Laser Center Lübeck, which is also responsible for the adaptation of the new OCT system to the surgical microscope. In addition, collaborations will also be developed on how to visualize 3-D volume data optimally in real time to the surgeon in order to continuously adapt the current tumor borders to the respective surgical procedure.
The project goal: to resect CNS tumors more radically in future using this new technology while at the same time protecting healthy brain tissue. The benefits: Preventing early tumor recurrence and improving quality of life for patients. This consists in a reduced number of recurrent surgeries leading to fewer hospital stays; The avoidance of neurological deficits makes it possible for patients to return to work or into the social environment earlier.
Paper:
Yashin K, et al. OCT-Guided Surgery for Gliomas: Current Concept and Future Perspectives.
Proceedings: